TheraMabs can offer the preparation of biological medicine research and development services, mainly for monoclonal antibodies, fusion protein and cytokines of Liquid injection and lyophilized powder for injection, including formulation design, Lyophilization cycle process studies, compounding and fill finish process studies, long-term stability and accelerated stability studies, provide fully automated final drug product formulation and filling under cGMP conditions as defined by the worldwide regulatory agencies including FDA, EMA, cFDA and agencies from other countries or regions such as Japan, Korea, Taiwan and india.
Formulation Development
Drug Product fill and finish
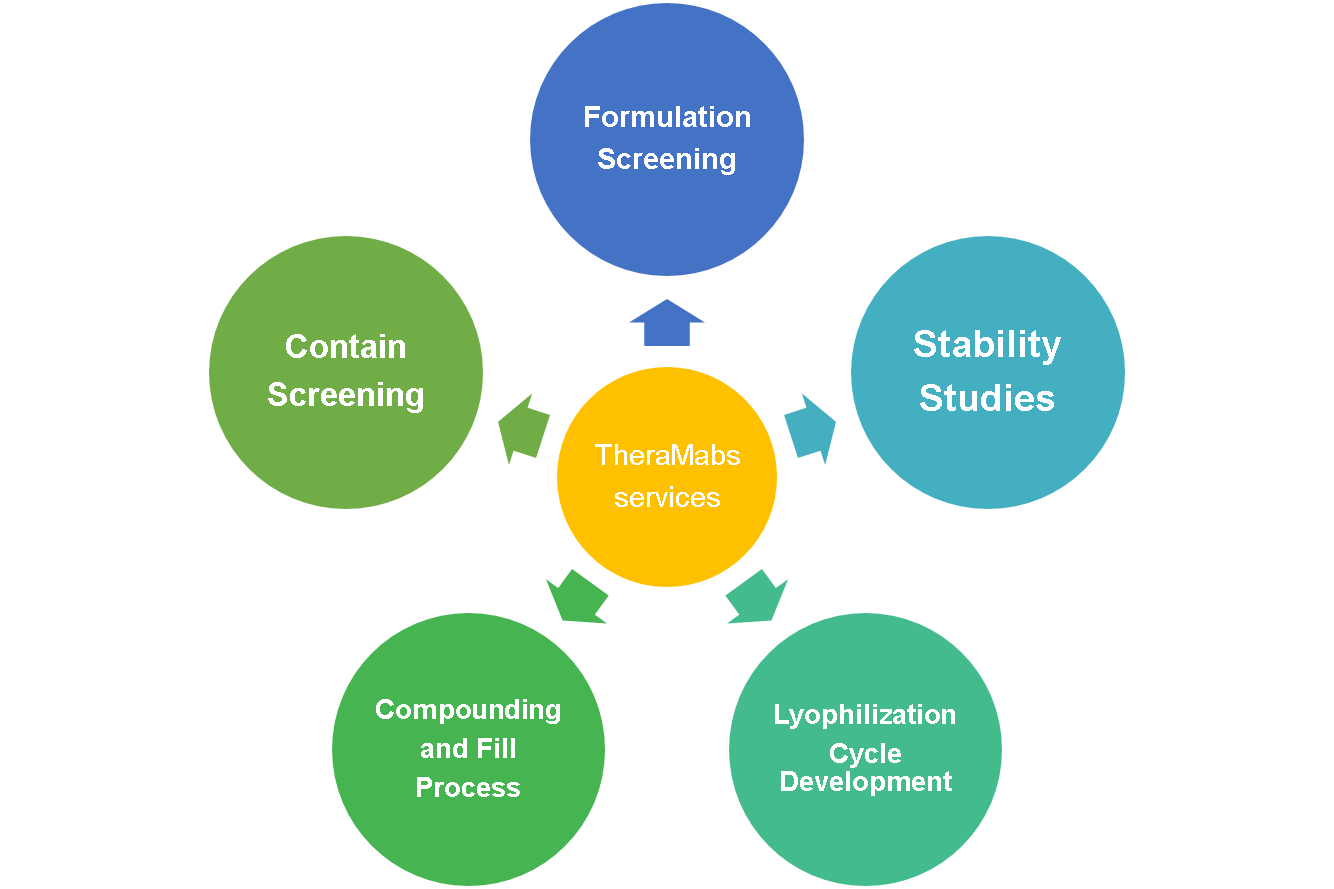
Formulation Development
(1) Formulation design
Rapid high-throughput screening of buffers and excipients utilizing Dynamic light scattering, Differential scanning calorimetry and spectrometry, Design of Experiment to identify optimal formulation and influencing factors, Forced degradation studies to identify degration pathways.
(2)compounding and fill finish process studies
Evaluation of addition order, mixing, concentration mechanisms, holding times, filtration, shear stress in filling, filling rate, crimping effect.
(3) Lyophilization cycle process studies
Screening freeze-dried protectant, filling agent, antioxidant, acid and alkali regulator, freeze-drying curve, etc.
(4) Stability studies
Long-term and accelerated stability studies under ICH guidance.
Drug Product fill and finish
(1) Both liquid and lyophilization formulation and fill.
(2) Dedicated formulation and filling facility for Biological agents drugs or other highly-potent drug products.
(3) Multiple validated container & closure configurations.
|