TheraMabs aims to promote the development of the
global pharmaceutical market and provide a real single source service with more
than 100 high-quality r&d talents, which is becoming a large-scale
pharmaceutical contract custom research and development production agency with
rich professional skills to help customers to achieve the project planning in
an efficient and economical way.
The service is as follows:
Cell lines Development
Cell culture process development
Purification process development
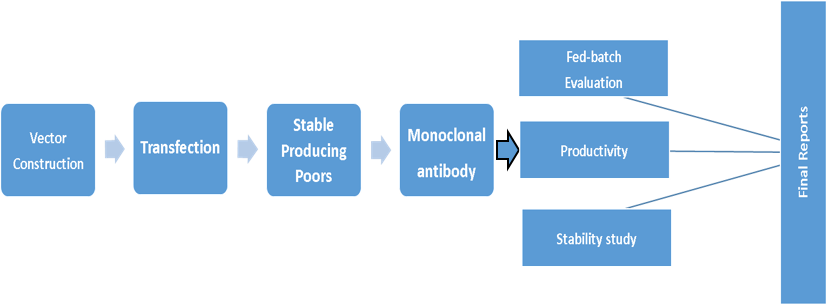
Cell Lines Development
TheraMabs can provide a variety of monoclonal antibody and fusion protein cell lines, according to the gene sequence provided by the customer, deliver high yield and stable expression of monoclonal cell lines on time.
Cell Lines Development Process
Platform features
(1) there are more than 50 cell lines of TheraMabs developed for use in clinical trials.
(2) professional cell lines technology platform with low licensing fees.
(3) high productivity(2~4g/L), good stability.
(4) professional cell banking and GMP cell banking research and development service (in accordance with FDA/ICH guidelines).
(5) suitable for monoclonal antibodies, fusion proteins, cytokines and other recombinant proteins.
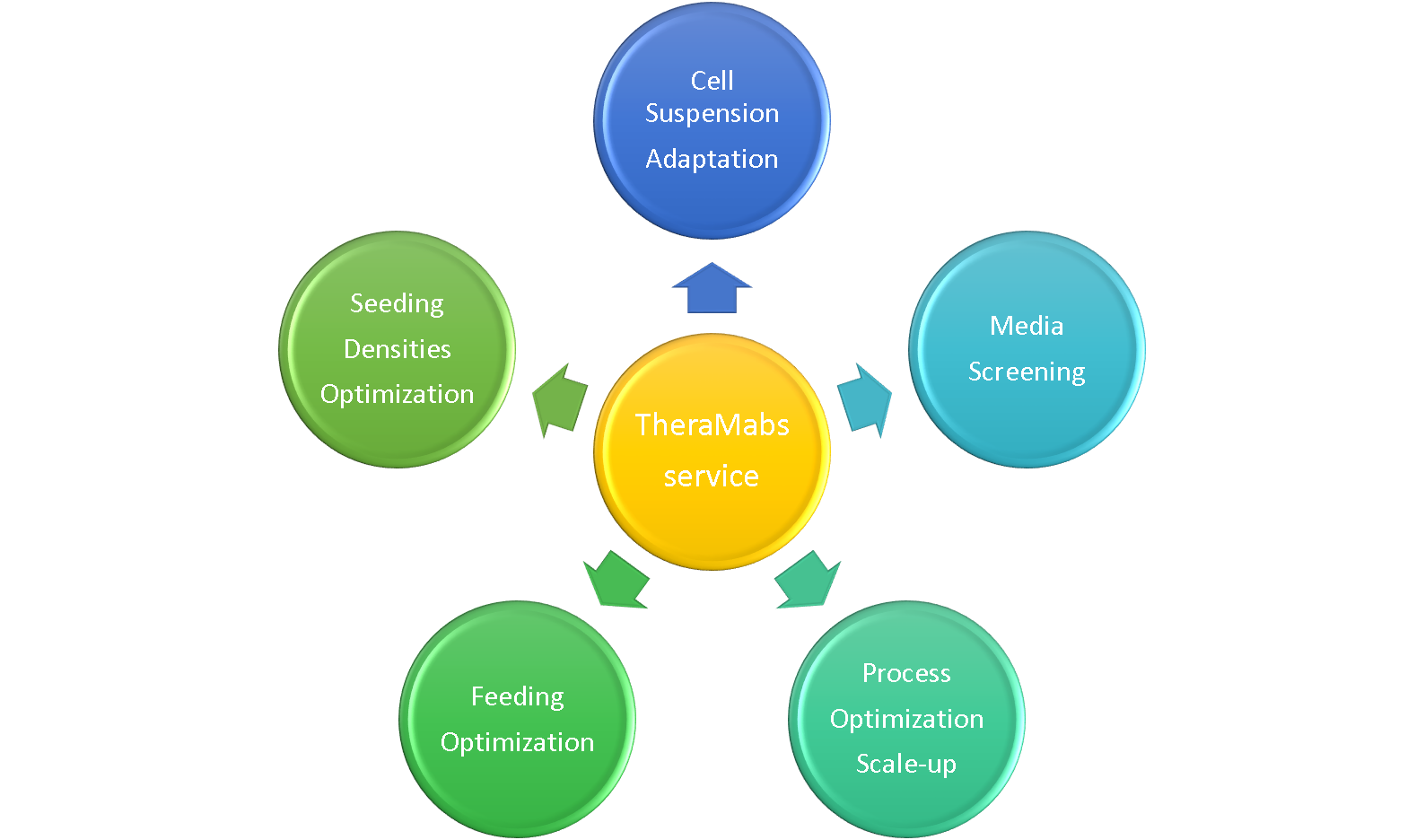
Cell culture process development
TheraMabs has an international first-class cell culture laboratory, GMP pilot workshop, and a team of experts engaged in cell technology development. Our cell culture process technology platform provides for adaptation of cell lines suspension culture, culture medium formula screening, feeding, cell inoculation density and cell culture technology parameters optimization for achieving maximum production, ease of scale-up. Our platform can run simultaneously more than 10 process development projects at different stages, and our cell culture development team can support service including:
Cell Culture Process
Independent project
(1) medium screening, adaptation of cell lines suspension culture, etc.
(2) Material generation in various scale bioreactors at different stages of projects, including sample production of the transient cell lines and stable cell lines.
(3) improving product quality (poly, charge isomer, glycosylation optimization, sialic acid content optimization, ADCC/CDC activity optimization) or improve production (2~4g/L).
IND-enabling cell culture process
(1) cloning screening, process optimization, scale-up.
(2) cGMP production for worldwide IND application submissions.
Purification process development
The purification technology development platform of TheraMabs has more than 10 sets of different purification system (AKTAprocess AKTApilot AKTAprime plus, deep filtration system, ultrafiltration and Diafiltration System), which can quickly and efficiently provide the required services for customers, provide a stable and repeatable and suitable for amplification of purification process to ensure that the maximum improve product purity and yield.
Available services include:
(1) filtration of the cell culture harvest material(depth filtration, centrifugation).
(2) chromatography resin screening.
(3) chromatography optimization (affinity chromatography, ion exchange chromatography, hydrophobic chromatography, mixed mode chromatography).
(4) studies on dynamic binding capacity of chromatography resin.
(5) study on the lifetime, aging and cleaning of chromatography resin.
(6) virus inactivation and virus removal process research.
(7) Scale-up research.
|